What role does health literacy play in clinical trial participation?

As part of our efforts to better understand patients and their motivations to participate in clinical trials, Antidote and SCORR Marketing partnered on a survey to gain a deeper understanding of how patients think about the medical research process. Survey participants were recruited by eight of Antidote’s partners, including the American Kidney Fund, Allergy & Asthma Network, Healthline, JDRF, Lung Cancer Alliance, Lupus Research Alliance, Melanoma Research Alliance, and the Multiple Sclerosis Association of America, which allowed Antidote and SCORR to obtain perspectives from nearly 4,000 patients with an array of conditions that could impact their thinking and behaviors.
Our survey results provide an in-depth perspective on patients’ motivations to participate in clinical trials. With these results, we are able to provide industry stakeholders with insights that will allow them to embrace patients as partners in clinical research and better understand how trial design and communication can impact a participant’s decision to enroll.
In this blog, we are focusing specifically on the health literacy aspect of clinical trial enrollment — we’ll consider both the importance of health literacy and how industry stakeholders can meet patients where they are to engage with them in a meaningful way, while providing key data to support our claims.
Why is health literacy important?
With 80 percent of internet users searching for health information online, digital channels have become an integral method for connecting patients to clinical trials. However, despite individuals’ willingness to seek health information, health literacy throughout the country remains low.
Low health literacy is not only costly, but it can also exclude underrepresented populations from research opportunities. According to John A. Vernon, PhD, at the University of Connecticut National Bureau of Economic Research (NBER), "Through all its impacts — medical errors, increased illness and disability, loss of wages, and compromised public health — low health literacy is estimated to cost the U.S. economy up to $236 billion every year." Additionally, it is vital to note that health literacy and health equity are intertwined concepts — providing translation services, plain-language informational materials, and testing messaging with intended audiences can all work together to create a more equitable healthcare system for everyone. Health literacy must be viewed in the context of language and culture in order to ensure the healthcare system is working for diverse populations.
Because of the cost and cultural impact, low health literacy slows clinical research and the development of new treatments and medical devices. It could be assumed that patients who do not understand the important process of clinical research are less likely to participate in clinical trials, and this hypothesis is borne out in our survey. Respondents who were more knowledgeable about clinical research were twice as likely to participate in a clinical trial than those who had less understanding.
It’s important to note that patients do want to improve their health literacy and learn more about clinical research. In our survey, 77% of respondents indicated they want the industry to make it easier to learn about clinical trials, 70% want findings from clinical trials more readily available, and 66 percent want clearer information about the costs of participating.
So how can the clinical research industry increase research knowledge? A key way is by partnering with online patient communities and advocacy groups, which can play an important role in improving health literacy and educating patients about clinical trials. An independent research study found that 57% of patients search for health information online first while 32% go to their doctor or other health care professional first.
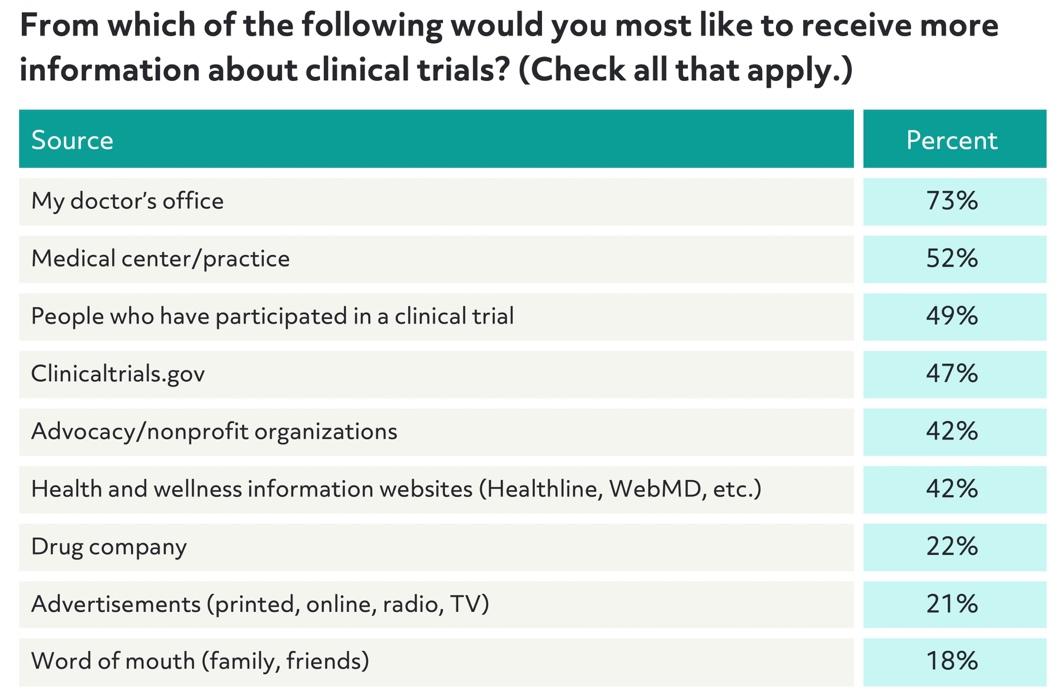
In our survey, respondents showed a clear preference for receiving information about clinical trials from their doctor or health care professional, 49% want to receive information from patients who have previously participated in a clinical trial, and almost twice as many preferred to receive information from an advocacy/nonprofit organization or health and wellness website (42%) than from a drug company or advertisement.
The FDA also sees the potential value in a more collaborative process and developed a new draft guidance in December 2018 that stated, "Patients, caregivers, patient and disease advocacy groups, and other stakeholders with knowledge of or access to the patient community, may be well-positioned to also make broader contributions to advance medical product development."
How to engage potential clinical trial participants
While partnering with advocacy groups, utilizing medical data, and doing digital outreach can all be helpful in finding patients who are potentially eligible, this does not necessarily guarantee those patients will be willing to partake in the study.
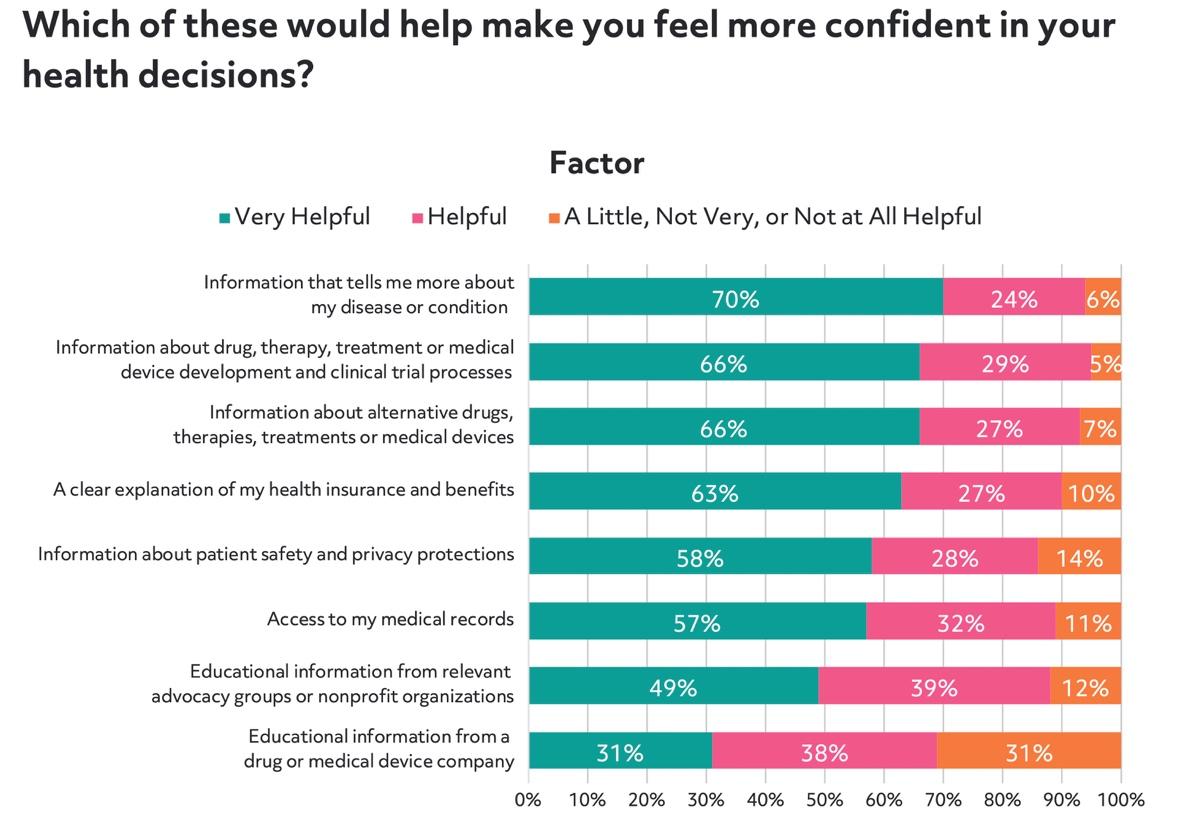
To get patients to the next step, our survey indicated that clinical research education, identifying specific patient needs, and empowering them to make personal healthcare decisions may be the critical bridge between finding patients and enrolling them in a study. Patients who exhibited less clinical trial knowledge in the survey expressed less confidence in their ability to make well-informed health decisions, like signing up for a clinical trial.
One helpful approach is to enable patients to take the next step quickly, while they are actively searching online for trials. Think With Google, a data resource site established by Google, backs up this approach, finding that 81 percent of smartphone users do searches on their phones that are more focused on the information they need immediately in order to take action.
Providing plain-language educational materials and an online pre-screening questionnaire can accommodate this "need it now" behavior and help move the patient forward. This same approach is equally suitable for caregivers of patients with low health literacy, cognitive impairment, or physical symptoms that can interfere with online or digital engagement.
As indicated above, patients in our survey prefer learning about clinical trials from medical professionals — a result consistent with earlier studies. However, this isn't where they actually learned about clinical trials.
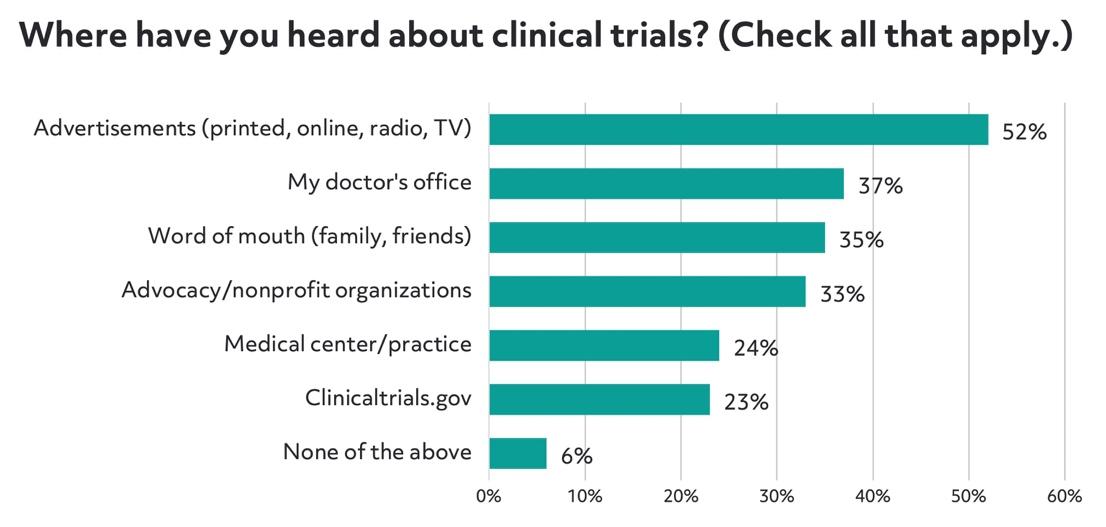
Advertisements were the most common way for patients to learn about trials, despite it being the second to last channel preferred by patients. Word of mouth was also cited by 35% of respondents, but it was the least preferred channel. While these sources were identified as where patients have heard about clinical trials, other channels should be explored. Sixty-one percent of those surveyed use message boards and health-based online communities to learn more about their condition and the experiences of other patients.
Similarly, when survey participants were asked about what would make them feel like a partner in clinical research, speaking with medical professionals directly was key. While online advertising can be improved to ensure clear communication with patients, our survey results suggest that it's most important to connect patients with real people as quickly as possible during the patient recruitment process.
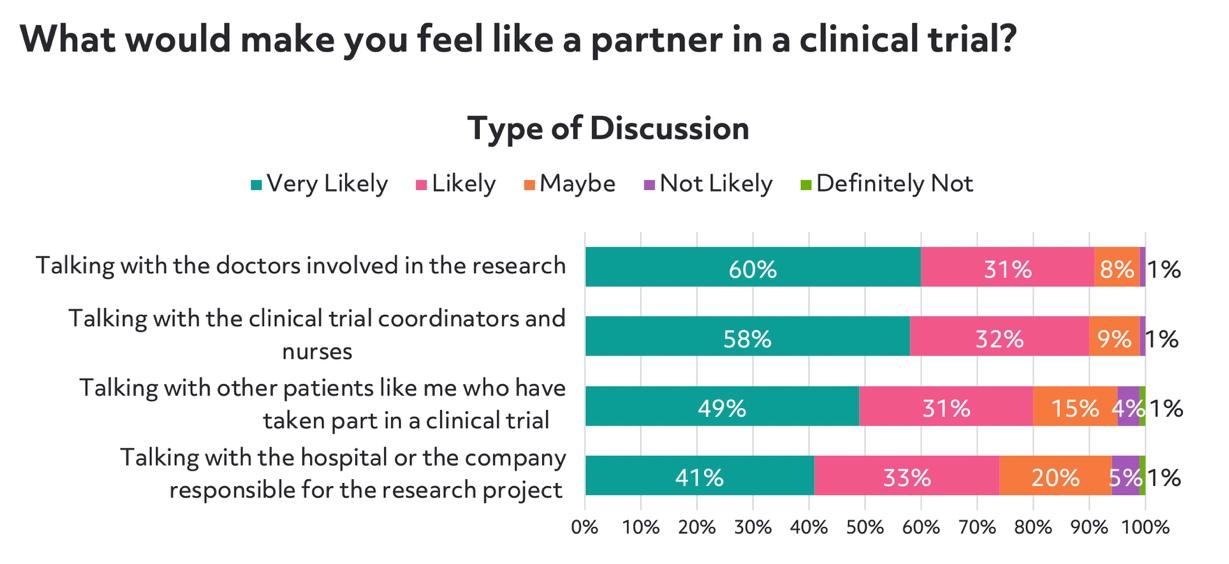
The survey findings, as well as actual online behaviors, point to the importance of helping a patient progress to the point of speaking with staff at a research site.
Once the patient connects to the research site, the process moves from online to offline, and patients can learn more about the specific study from the people they want to learn from: medical professionals. However, providing strong outreach materials, online pre-screeners to give patients agency, and highly trained staff to follow up on their survey responses can help them get to that step more confident and prepared to make their next decision.
Topics: For Sponsors
