7 factors that impact clinical trial patient recruitment

Participating in a clinical trial is a significant decision for patients, and several factors can influence their decision to enroll. These factors include understanding the trial's details, goals, and logistics. Sponsors must understand what matters most to patients when designing protocols, and prioritizing patient-centric considerations is key to enhancing patient engagement and trial success.
That's why Antidote asked nearly 4,000 patients about their views on participating in clinical trials. Successful patient recruitment keeps the patient perspective throughout the entire process, which includes protocol, site performance, and communication. Below, we'll share how to optimize a study design to prioritize patient retention strategies at each stage of a clinical trial.
Factors that influence patient recruitment for clinical trials
Clinical trial eligibility criteria
While eligibility criteria are important for establishing accurate data, overly-stringent criteria can make it difficult to find qualified patients for a study. By streamlining criteria to remove information that does not impact participant safety or that isn't related to primary research questions, trial sponsors can improve inclusivity without compromising the study results.
Additionally, strict eligibility criteria can also reduce diversity among clinical trial participants. The FDA recently issued guidelines on enhancing the diversity of clinical trial populations, specifically focusing on eligibility criteria, enrollment practices, and trial design. Their guidance notes that some common exclusions have less scientific backing, such as excluding those at extremes of the weight range, those with organ dysfunction, and those with HIV. The FDA recommends broadening these eligibility criteria when appropriate to reach a more diverse patient population.
The FDA also suggests moderating exclusion criteria that may be scientifically sound, but too broad. For example, heart disease studies may exclude those with severe heart failure, but those with more mild disease may be able to participate without repercussions. Considerations like these can make it easier to have the participant pool of the study truly reflect real-world conditions, which means if the treatment is approved, it is more likely to work as intended for the target population.
The patient need for alternative therapy options
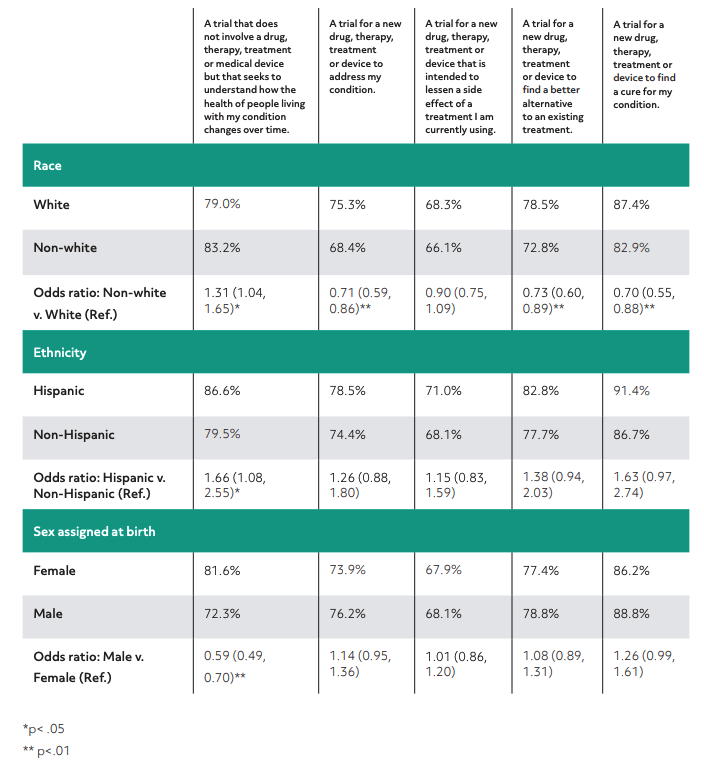
Our research found that patients are very interested in knowing the goal of a clinical trial, and preferred certain types of goals over others. The survey respondents were most interested in clinical trials researching a cure for their condition or a new treatment option and were less interested in trials researching treatments for side effects of other existing medications. Therefore, when designing patient recruitment materials, it’s important to highlight meaningful symptoms or challenges that the trial is meant to address. If a trial fails to address the concerns that are most important to patients, it may be worth reexamining the protocol.
The chart below shows the frequency of respondents who answered “likely” or “very likely” to partake in the specified type of trial by demographic categories and odds ratios of responses by demographic information (referent group indicated as “Ref.”).
Site locations
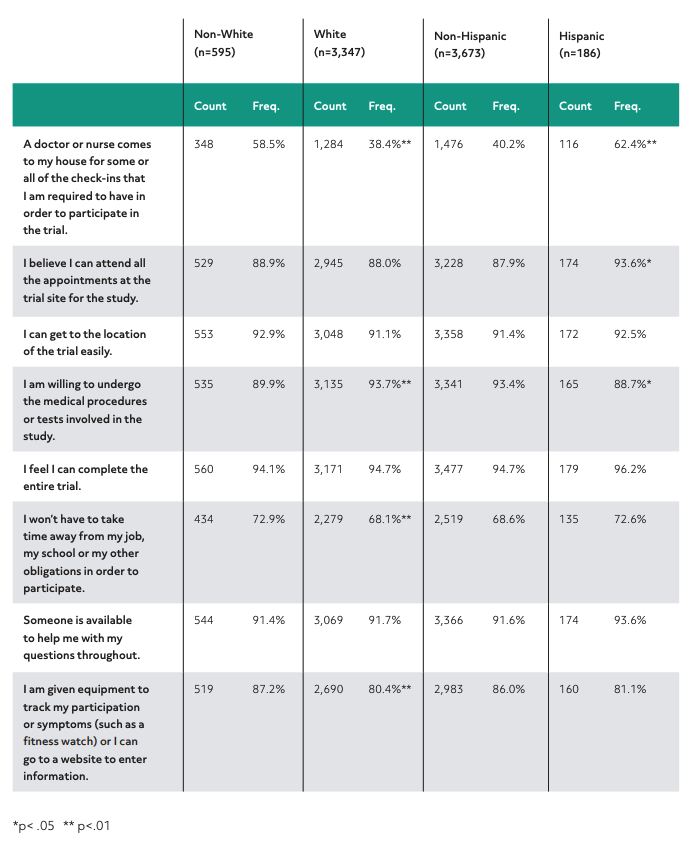
When selecting sites for a clinical trial, it’s important to opt for sites located in areas with significant patient populations. The problem is that this excludes patients, many of whom are people of color or who have low incomes. The assumption is that the logistics would discourage participation but our research discovered that providing transportation or virtual site visits could expand the patient participation pool and help bring in more diverse participation.
This chart illustrates the count and frequency by race and ethnicity of respondents who answered “likely” or “very likely” to the question: If you were considering taking part in a clinical trial, how important would the following be to you? (Please note: Non-white and non-Hispanic individuals served as the referent group for statistical analysis.)
In the aftermath of the COVID-19 pandemic, many sponsors are rethinking what it means to have a site at all, and many researchers have traded traditional processes for virtual or decentralized opportunities. One major advantage of decentralized trials is that they remove the burden from patients and encourage participation from wherever patients may be, all while minimizing health risks for patients taking part. Consider adding elements of decentralized trials to your protocol.
Patient follow-up services
Once trial enrollment starts, site performance can have a major impact on achieving randomization numbers. Even if a site is in the ideal location, factors such as inexperienced staff or poor training can hinder the patient recruitment process and potentially discourage patient participation. Before starting recruitment, ask sites what would help them be successful in their efforts, and consider working with a clinical research recruitment company that offers follow up services to reduce site burden.
Seasonality
Seasonality should be taken into consideration when developing a patient recruitment plan. Seasonality can include both the weather patterns at specific site locations, as well as seasonal events such as holidays. Symptoms may for some conditions can worsen during different times of the year, making patients more likely to seek out a clinical trial opportunity. When creating a patient recruitment plan, account for seasonal dips in enrollment.
Patient recruitment resources
Before launching a clinical trial, it’s important to be sure that sufficient funds have been budgeted to cover effective clinical trial recruitment strategies. A clinical trial recruitment rate calculator can help sponsors estimate how many patients should come from each site per month and then estimate what support sites will need to reach those numbers. Getting this right is important — generally, including patient recruitment services early on can help reduce costs over the course of a clinical trial, as opposed to only seeking "rescue" support at the last minute.
Messaging materials
Even the most easily accessible, well-designed clinical trial needs compelling outreach materials and the expertise of a patient engagement agency. Before choosing a patient recruitment agency, ask to see successful outreach materials they've created in the past. The best advertisements capture the patient experience and make sure to highlight benefits and goals that resonate with each patient population. When used strategically, outreach materials can help assuage several of the challenges included in this list by highlighting the benefits of taking part.
Want to learn more about how targeted recruitment plans can lead to time-saving results? Download our patient recruitment case studies.
Topics: For Sponsors
