Advertising Clinical Trials: What Works in 2019

There are more approaches to advertising clinical trials than ever before, from digital advertising, to site databases, to sharing materials in doctors' offices. Unfortunately, trial protocols have become more complex at the same time outreach options have expanded. Despite the best efforts of the industry, 73% of Americans don't even know anyone who has participated in a trial, let alone joined one themselves.
That means that today more than ever, clinical trial patient recruitment requires a diverse and comprehensive approach that puts trial participation into context for patients. Below, we've outlined the principles that are most effective at clinical trial recruitment today.
What works: Including digital in your outreach from the start.
Traditionally, digital advertising has been seen as a backup plan by trial sponsors, rather than an approach to include in initial outreach. While digital advertising may be associated with high upfront costs, including it in your plan from the beginning can actually help reduce costs in the long run. For example, most studies plan to only recruit from research site databases – as recently as 2014, only 11% of drug trials used social media advertising for patient recruitment. When that approach falls short, sponsors may request support from a patient recruitment company for a "rescue" trial. This approach often ends up being more expensive because the recruitment company has less time to learn about top-performing content and targeting. With a constricted timeline, it's also more challenging to make adjustments based on bottom-of-the-funnel results.
As study protocols have become more and more complex, it's become increasingly challenging to recruit exclusively from patient databases. Digital outreach allows patient recruiters to reach out to more targeted populations and iterate during a campaign to improve results.
What works: Driving your strategy with data.
An experienced patient recruitment company will bring data from prior trials, patient surveys, and trial-specific research to bear on their outreach strategy. This approach helps recruiters find the right patients for a trial faster, rather than casting a wide net and connecting with ineligible or uninterested patients.
At Antidote, we drive our patient recruitment strategy with both quantitative and qualitative research based on clinical trial searches through our partners, conversations with patients, and dedicated research on each patient population.
Last year, for example, Antidote conducted a survey of 4,000 patients with some of our nonprofit partners and SCORR Marketing. Through that survey, we found that what matters to patients in clinical trials varies by condition area. For example, while logistics and transportation support is often cited as key to improving participation rates, in our survey, participants ranked transportation last in terms of importance. Preferences also varied by condition area. We use this data to inform what trial benefits to highlight when creating outreach materials for different clinical trials.
What works: Keeping the "buyer's journey" in mind.
While clinical trial participants aren't technically buying anything, the buyer's journey is still a helpful model for thinking about how patients learn about and consider clinical trial participation. For example, a recruitment company may create an email outreach campaign or ad that lays out the problem (the "awareness" stage), offers the trial as an option to solve it, and outlines benefits to participating in the trial.
The buyer's journey is also useful to think of when developing retargeting or follow-up campaigns. Joining a clinical trial is a big decision for patients, and patients may need to see information about your trial a few times before deciding to take the next step.
What works: Meeting patients offline, too.
There are more ways than ever to connect with patients digitally, from social media advertising to matching with patients through electronic health records. It's critical to remember, though, that behind every screen sits a person with individual health needs and motivators for participating in research.
Last year, Antidote conducted a survey of 4,000 patients that found that most patients learned about clinical research opportunities online:
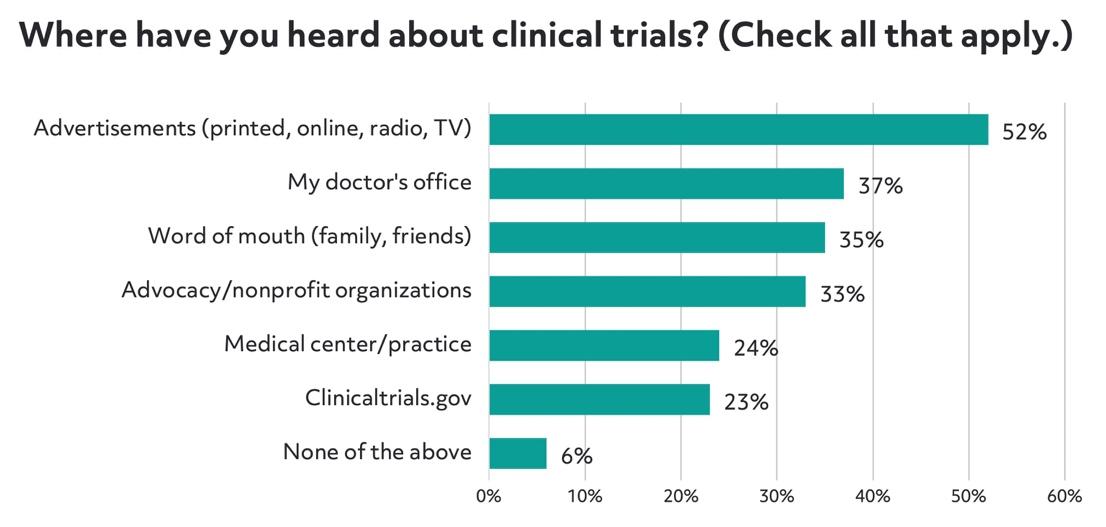
However, patients prefer to learn about clinical trials from their doctor. It makes sense that patients would be interested in getting their doctor's seal of approval on participating in a clinical trial, but many doctors don't have the time or resources to proactively share information with patients:
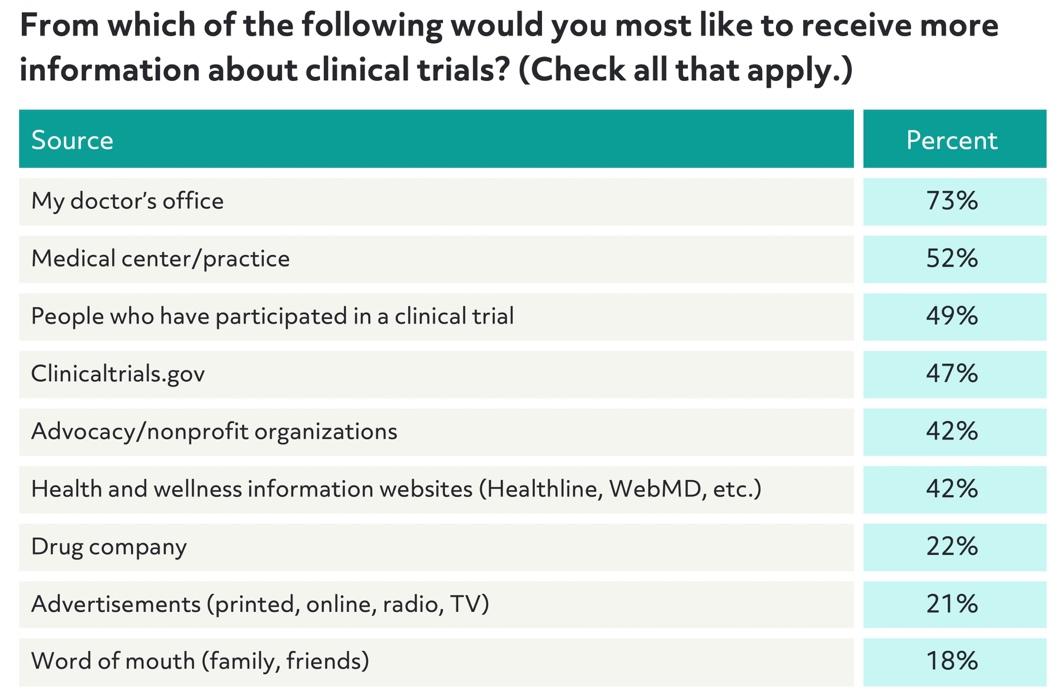
Meeting patients offline, whether through partnerships with nonprofits and advocacy groups or through relationships with doctors and hospitals, can help patients feel more comfortable with trial opportunities. In person, patients are better able to ask questions and learn about a trial in real time, while hearing about options from sources they trust.
What works: Using a range of clinical trial advertising services
Clinical trial recruitment companies offer a range of advertising services that can help speed up enrollment for your trial. A diverse approach can help you reach your clinical trial patient recruitment goals faster.
Campaign management and optimization.
When done well, running ads on platforms like Facebook and Google is a full-time job. For best results, marketers will closely track how their chosen audiences are responding to their ads and make adjustments throughout a campaign. Often, marketers will manage several different tests before settling on the best-performing ad and targeting. Clinical trial advertising agencies may also have access to lookalike audiences or experiences from prior trials and can apply that data and those findings to new trials.
Landing page or pre-screener creation.
In addition to providing ad copy and design, some recruitment companies will also create dedicated landing pages to tell patients more about your trial and guide them to the next step. You may choose to have patients answer a few questions online to begin the screening process, or direct patients to either call a research site near them or send an email to learn more.
Clinical trial patient recruitment companies may also offer research site follow-up services to continue supporting the patient journey and track which patients make it to research sites for further screening. This process can also improve outreach, as advertisers can prioritize top-performing research sites.
Design development.
Some patient recruitment companies also specialize in ad design, which can make a significant impact on performance. High-quality design also creates an intuitive user experience that helps more patients express interest in your trial. Small details like choosing photography that reflects your patient population also make a significant difference.
Copywriting.
In clinical trial patient recruitment, ad copy should engage patients while following strict IRB and FDA guidelines. It can be a tricky balance to strike, and making multiple submissions to an IRB because your materials aren't compliant can slow your trial timeline. Working with an experienced clinical trial advertising agency can help. A quality agency will also provide plenty of copy options for a range of ad placements and tests so your team can optimize your campaign.
Partnership support.
Clinical trial recruitment companies can also offer access to additional outreach options beyond traditional ad platforms. Health nonprofits, patient advocates, and community groups may have relationships with clinical trial recruitment companies to help your trial reach niche groups of patients and connect through in-person events or other personalized approaches.
If you're interested in using any of these clinical trial advertising services for your trial, download our case studies to learn more about us.
Topics: For Sponsors

