How to Optimize Patient Recruitment in Five Steps

Of all of the elements of a clinical trial, optimizing patient recruitment may make the most significant impact on trial timelines. Even if you're meeting your recruitment goals – a rare accomplishment, to say the least – trials and budgets always benefit from better efficiency.
To optimize patient recruitment, it helps to keep a close eye on how sites are performing, whether you're getting the results you're looking for from patient recruitment companies, and if any improvements can be made once patients express interest in a trial.
These five steps can help optimize patient recruitment:
- Identify low-performing research sites and consider reallocating resources.
- Look for weaknesses in outreach materials.
- Find what approaches are working well and reallocate resources if possible.
- Fix the patient journey.
- Ask research sites and patient recruitment companies for feedback.
We'll get into how to take each step below.
Step 1: Identify low-performing research sites and consider reallocating resources.
If you're behind on your recruitment goals, it can help to examine which research site locations are on track, and which are struggling.
Start by calculating your ideal recruitment rate per month and per site, then identifying which sites are falling short. Then, you can request that your patient recruitment company reallocate resources to higher-performing sites. If the agency is using geo-targeting in their outreach, it's simple enough to direct more referrals toward sites that are performing well.
Diversity is important in clinical trial recruitment, and if this approach threatens the geographic or demographic makeup of your trial, more resources may need to be allocated to send referrals to those sites. For example, if the site is in a rural area, the trial may need to offer transportation support to help patients take part.
Step 2: Look for weaknesses in outreach materials
Your trial outreach materials are another piece of the recruitment puzzle that can impact your results. Without engaging advertising materials or invitations, patients simply won't be interested in taking part.
When evaluating materials provided by sites and patient recruitment companies, ask whether the outreach materials for your trial answer common patient questions.
The best clinical trial advertising strikes a balance between engaging, creative content and practical, actionable information. A clinical trial recruitment company experienced in digital marketing will provide a suite of creative materials to test. Messaging variations in clinical trial advertisements could include:
- The benefits of taking part in the trial. While continuing to follow FDA clinical trial advertising guidelines, recruitment materials can still share some of the benefits of joining a trial, such as receiving high-quality care from experts.
- The goal of the trial. In research Antidote conducted, patients were – understandably – most interested in clinical trials that were researching a potential cure for their disease, or a new treatment to extend their life. They were less interested in trials researching treatments for side effects of existing medications. Outreach materials should share the goal of the trial and how it will directly impact quality of life.
- The science behind the trial. In our research, we found that patients are most interested in hearing directly from the doctors and nurses involved in a research project. Of course, science is complex, and it's important to share materials that patients with low health literacy can understand, too. At the same time, consider including patient-friendly details about how researchers believe the treatment may work. Study staff should also be prepared to answer detailed questions about the science behind the trial during the informed consent process.
- Answers to frequently asked questions. For example, patients often ask whether or not a trial will use a placebo. Rather than obfuscating information that may deter patients from taking part, consider sharing trial details up front.
Ideally, your recruitment companies and sites will have enough materials on hand to test additional creative options without re-submitting to your IRB. If you have to re-submit, encourage recruiting teams to keep their outreach material packets small and targeted to expedite the process.
Step 3: Find what's working well, and make the most of it.
Even if your recruitment schedule isn't where you'd like it to be, take a look at where successful referrals are coming from. Ask your research team:
- Is there a top-performing research site with a robust database that's leading in randomizations?
- Are you working with a patient recruitment company that's sending high-quality referrals?
- Are there doctors or medical facilities interested in sharing more referrals for the trial?
- Can we allocate more resources to recruitment referral sources that are going well?
You can also analyze whether patients from certain sources are more or less likely to meet any challenging inclusion/exclusion criteria the trial may have. For example, you may find that patients coming from a site's database are experiencing symptoms that are too severe for the trial. Identifying where issues are and where the work is going well can help you optimize your recruitment efforts.
Step 4: Fix the patient journey.
If recruitment is lagging, look for issues in your patient flow. Are you getting plenty of referrals, but not enough in-person screenings at the site?
It's easy for research sites to become overwhelmed by patient referrals – in fact, 34% of referrals are never processed by research sites, according to research conducted by PAREXEL. Consider working with a clinical trial recruitment company that screens patients on the phone and helps them take the next step, if this has become an issue for your trial. These groups can also help you screen patients over the phone so sites have fewer, more qualified patients to work with. Recruitment companies can also help connect sites with patient medical records, share blood test results, and complete other tasks that ease the burden on sites.
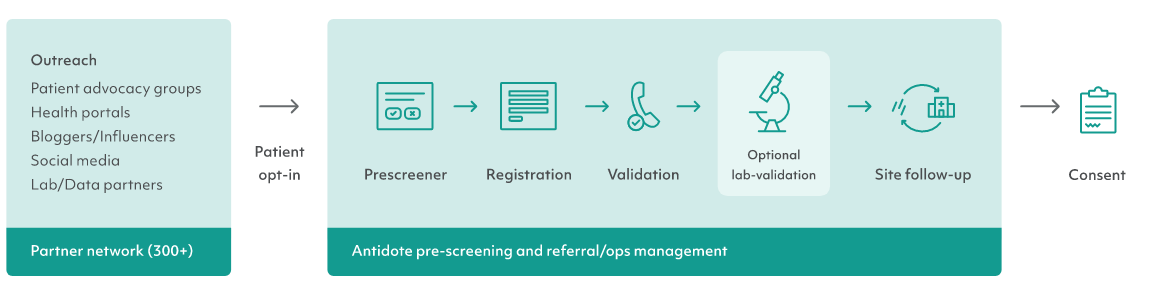
For example, Antidote offers phone validation, lab validation, and site follow-up services to support research sites.
Step 5: Ask research sites and patient recruitment companies for feedback.
Those who are on the ground level in patient recruitment have the most helpful perspectives on why a clinical trial may be running into delays. Set up a feedback loop to hear from recruitment companies and research sites about issues with the protocol. If possible, consider re-evaluating the inclusion and exclusion criteria for the trial, and including a protocol amendment if scientifically feasible.
Looking for more advice on how to optimize patient recruitment? Download our whitepaper on precision recruitment.
Topics: For Sponsors

